SPECIFICANCER
Meet SPECIFICANCER, whose challenge was to devise approaches to prevent or treat cancer based on mechanisms that determine tissue specificity of some cancer genes.
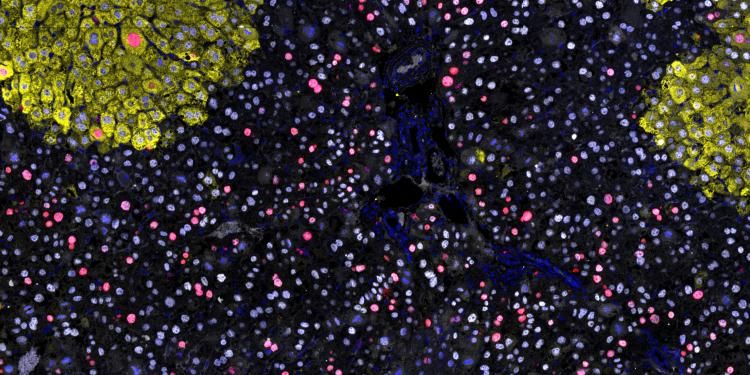
SPECIFICANCER took on our tissue specificity challenge, to understand why despite being expressed in a wide variety of tissues, some mutations drive tumorigenesis only in certain tissues. Today, the team published its latest work in Nature, led by Owen Sansom at the Cancer Research UK Scotland Institute.
Here, first author and SPECIFICANCER Future Leader Alex Raven takes us through the work. The team identified zonal differences within the liver that determine the ability of Wnt to drive tumourigenesis and explain why different Wnt pathway mutations vary in their tumourigenic potential, revealing new ways to therapeutically target this pathway.
Genetic mutations to oncogenes and tumour suppressor genes drive cancer, but what we do not understand is why specific mutations accumulate in certain cancer types and not others. For example, mutations to the Wnt signalling pathway are common in colorectal and liver cancer but rarely occur in renal cancer or malignant brain tumours.
The liver is particularly puzzling as the normal role for Wnt pathway signalling is to stimulate cell differentiation, a feature not favourable to oncogenesis. By understanding the key factors in an organ that enable genetic alterations to form cancer and conversely the mechanisms that prevent other types of mutations, we can develop new, alternative, strategies to combat cancer.
We used genetically engineered mouse models to activate different types of Wnt mutations, sporadically, across the liver and then monitored their tumourigenic potential. A range of Wnt mutations were used; some mutations, such as β-catenin alterations, are regularly found in liver cancer, while others, for instance the loss of the tumour suppressor Apc, are infrequently detected in liver cancer patients.
Surprisingly all Wnt mutations were ineffective at causing cancer in the mouse liver. By exploring patient data sets our SPECIFICANCER team mates in the Park lab at Harvard Medical School identified the co-occurrence of Myc gene copy gain in Wnt-mutant liver cancer. We therefore included a Myc over-expressing transgene in our Wnt-mutant cancer model and discovered a subset of β-catenin-mutant clones formed tumours.
However, a large number of mutant clones still failed to progress to cancer. Through the use of spatial transcriptomic technology we found that the mutant-tumours were less differentiated than their neighbouring mutant-single clones.
We further examined the role of cell differentiation in tumourigenesis and noticed that the default response to Wnt mutations in the liver is differentiation to a particular hepatic-zonal fate. Mutant cells in this zonal state failed to form tumours as they lacked key pro-growth pathways (ie. mTOR signalling) and a proliferative translatome needed to form tumours.
The clones that did manage to progress were exposed to external signals that could reverse cell differentiation, enabling them to activate mTOR signalling, increase RNA translation and protein synthesis.
Finally, we re-examined the Apc mutant mouse livers and discovered that Apc loss strongly activated the Wnt pathway in liver cells locking them in a differentiated state that they cannot escape, which prevents their progression to tumours and explains why these type of mutations are not common in patients.
Looking forward, this discovery is exciting as it reveals new strategies that could be used to treat a historically difficult oncogenic signalling pathway. We identified key vulnerabilities, such as mTOR signalling, that could be targeted to indirectly suppress Wnt-driven liver cancer and prevent tumour formation.
I completed my PhD at the University of Edinburgh, studying tissue regeneration and stem cell biology with Stuart Forbes. It was during this time that I became interested in how diseased tissues alter cell fate and identity. I therefore undertook a post-doctoral transition fellowship to move across Scotland and study cancer cell plasticity in the group of Owen Sansom at the CRUK Scotland Institute.
It was here where I had the fantastic opportunity to join the SPECIFICANCER team and investigate Wnt pathway mutations across a range of cancers. Earlier this year, I was awarded the prestigious UKRI Future leader fellowship which will support me over the next 7 years to establish my lab and investigate how chronic damage alters liver homeostasis and enables mutant clone expansion and tumour formation.
Through Cancer Grand Challenges team SPECIFICANCER was funded by Cancer Research UK and The Mark Foundation for Cancer Research.
Top image caption: Oncogene driven proliferation (red marker) predominantly occurs in cells that are not differentiated to a zone-3 fate (yellow marker). Credit: Alex Raven
Meet SPECIFICANCER, whose challenge was to devise approaches to prevent or treat cancer based on mechanisms that determine tissue specificity of some cancer genes.
Learn about our Future Leaders, poised to redefine the landscape of cancer research.